Which Best Describes the Hydrogen Bonding Between Two Water Molecules
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions and is the primary interaction occurring in ionic compoundsThe ions are atoms that have lost one or more electrons termed cations and atoms that have gained one or more electrons termed anions. Relate the interaction potential to the forces between molecules.
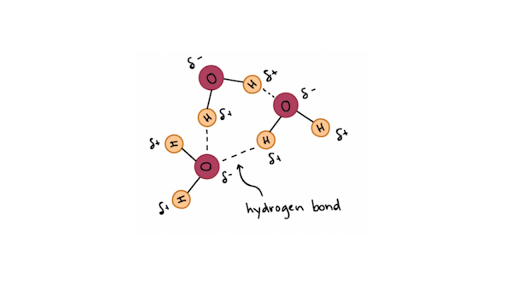
Hydrogen Bonds In Water Article Khan Academy
Watch different types of molecules form a solid liquid or gas.

. This describes the way by which polypeptides are coiled. 13 Oxygen nitrogen and fluorine bond with hydrogen to form molecules. Change the temperature or volume of a container and see a pressure-temperature diagram respond in real time.
This transfer of electrons is termed electrovalence in contrast. This structure is formed as a result of the hydrogen bonds between carboxyl and amine groups in close amino acid monomers. Add or remove heat and watch the phase change.
The two main types of secondary structure are the α-helix and the ß-sheet. This describes the three-dimensional shape of proteins. These molecules are attracted to each other by a coordinate covalent bonds c ionic bonds b electrovalent bonds d hydrogen bonds 14 The bond between hydrogen and oxygen in a water molecule is classified as a covalent and nonpolar c ionic and polar.

Hydrogen Bonding Chemistry For Non Majors
How To Describe The Hydrogen Bonding Between Molecules In Water Quora

No comments for "Which Best Describes the Hydrogen Bonding Between Two Water Molecules"
Post a Comment